3 月 . 07, 2025 07:07 Back to list
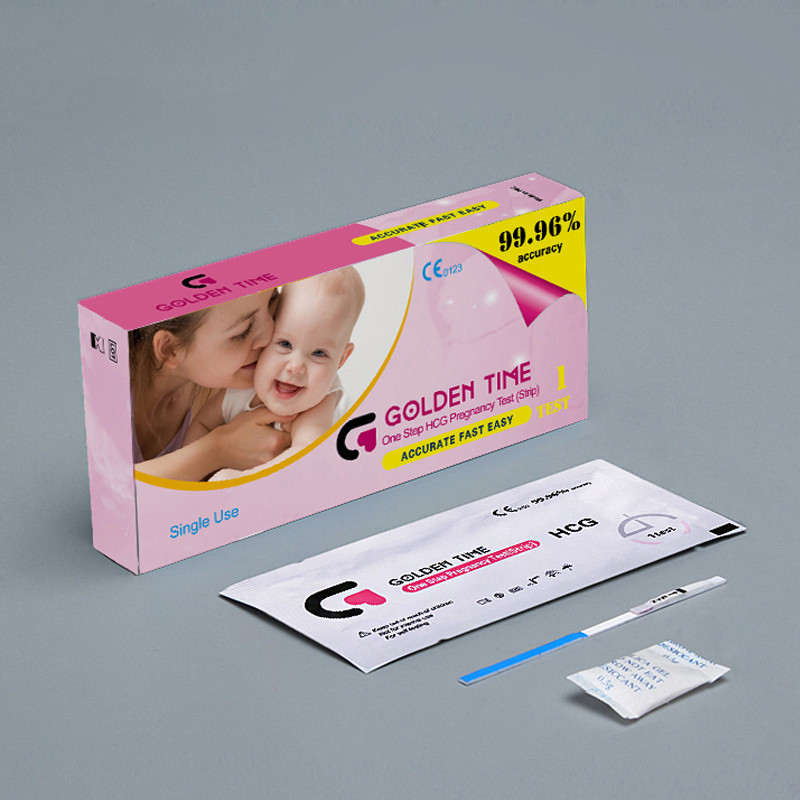
Pregnancy Test Empty Cassette
Dengue fever remains a pressing global health concern, particularly affecting tropical and subtropical regions. The rise in dengue cases necessitates reliable and efficient diagnostic methods. As such, the role of dengue test manufacturers is pivotal in managing this disease’s spread and providing timely and accurate diagnosis. Industry leaders in developing dengue diagnostic tools focus on four main metrics crucial for product success experience, expertise, authoritativeness, and trustworthiness.
Authoritativeness in the industry is often measured by the ability to secure certifications from global health bodies. Manufacturers that consistently achieve ISO certifications, as well as approvals from the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA), establish themselves as authoritative voices in the field. These certifications are not mere formalities; they signify that the products have undergone rigorous testing and conform to international safety and efficacy standards. This authoritativeness not only reassures healthcare providers and patients of the test's reliability but also strengthens the manufacturer’s market position. Trustworthiness, a crucial factor in user acceptance, stems from a transparent development process and a solid track record of performance. Manufacturers that maintain open channels of communication about their product’s capabilities and limitations create a foundation of trust. Customer testimonials, case studies, and peer-reviewed research publications further substantiate a test’s credibility. By fostering strong partnerships with hospitals and research institutions, these manufacturers can gather ongoing feedback and swiftly address any issues, further cementing their reputation as trustworthy entities in the healthcare ecosystem. The indispensable role of dengue test manufacturers in global health systems underscores their responsibility to uphold these four metrics. Products from trusted manufacturers help contain and manage dengue outbreaks, ultimately saving lives. Their efforts in producing accurate, efficient, and accessible diagnostic tools contribute significantly to the broader fight against vector-borne diseases. As advancements in technology continue to revolutionize the diagnostics industry, these manufacturers remain at the forefront, adapting to new challenges and setting benchmarks for quality and safety in dengue testing.
Authoritativeness in the industry is often measured by the ability to secure certifications from global health bodies. Manufacturers that consistently achieve ISO certifications, as well as approvals from the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA), establish themselves as authoritative voices in the field. These certifications are not mere formalities; they signify that the products have undergone rigorous testing and conform to international safety and efficacy standards. This authoritativeness not only reassures healthcare providers and patients of the test's reliability but also strengthens the manufacturer’s market position. Trustworthiness, a crucial factor in user acceptance, stems from a transparent development process and a solid track record of performance. Manufacturers that maintain open channels of communication about their product’s capabilities and limitations create a foundation of trust. Customer testimonials, case studies, and peer-reviewed research publications further substantiate a test’s credibility. By fostering strong partnerships with hospitals and research institutions, these manufacturers can gather ongoing feedback and swiftly address any issues, further cementing their reputation as trustworthy entities in the healthcare ecosystem. The indispensable role of dengue test manufacturers in global health systems underscores their responsibility to uphold these four metrics. Products from trusted manufacturers help contain and manage dengue outbreaks, ultimately saving lives. Their efforts in producing accurate, efficient, and accessible diagnostic tools contribute significantly to the broader fight against vector-borne diseases. As advancements in technology continue to revolutionize the diagnostics industry, these manufacturers remain at the forefront, adapting to new challenges and setting benchmarks for quality and safety in dengue testing.
Latest news
-
Early Pregnancy Test Kits Accurate & Fast Results Bulk Order Now
NewsMay.30,2025
-
Buy OPK Tests for Pregnancy Detection Bulk Supplier Discounts
NewsMay.30,2025
-
Buy OPK Tests for Pregnancy Detection Bulk Supplier Discounts
NewsMay.30,2025
-
Best At Home H Pylori Test Kits Accurate, Fast & FDA-Certified
NewsMay.29,2025
-
Accurate Syphilis Test Kits Trusted Suppliers & Manufacturers
NewsMay.29,2025
-
Wholesale Stool Occult Blood Test Kits Bulk Supplier Pricing
NewsMay.29,2025

