News
-

Rapid test-What Are Sustainable Alternative Materials for Biodegradable Rapid Test Kit Cassette?
Today, single-use plastics are still widely utilized for the production of many medical and diagnostic devices despite their detrimental environmental impact. Increased use of plastics in COVID-19 test kits and personal protective equipment throughout the last two years has set the world back in its struggle against plastic waste. Indeed, according to the International Solid Waste Association (ISWA), single-use plastics may have increased as much as 300 percent since the start of the COVID-19 pandemic, and it is estimated that every single time that a COVID-19 lateral flow test is performed, 10 grams of plastics is thrown away into the environment.Read more -

Rapid test-Plastic Cassette For Rapid Lateral Flow Test
Plastic Cassette For Rapid Test offer ease and safety in diagnostic testing. These cassettes are compact, easy to use, and designed for single-use to prevent sample cross-contamination. They feature a clear window for easy result readout and can be used with a variety of samples, including whole blood, serum, and urine. Our cassettes are made from high-quality materials that ensure reliable and consistent test performance. The reagent pad is placed evenly and precisely to ensure accurate results. Additionally, our lateral flow cassettes are fully customizable to meet the specific testing needs of our clients. Overall, our quality lateral flow cassettes are an ideal solution for accurate, efficient, and safe diagnostic testing.Read more -

Medical devices-Modern Medical Devices: Technological Innovation and Healthcare Revolution
The history of medical devices can be traced back to the earliest practices of human medicine. Ancient tools such as surgical knives and forceps, though rudimentary, laid the foundation for medical advancements. Over time, developments in mechanics, electronics, and computer science paved the way for innovative medical devices.In the mid-20th century, the introduction of X-ray imaging technology marked the beginning of a new era in medical imaging. Subsequent advancements, including ultrasound, magnetic resonance imaging (MRI), and other imaging technologies, provided healthcare professionals with more accurate and comprehensive patient information. Concurrently, the development of internal implantable devices, such as pacemakers and artificial joints, extended the lifespans and improved the quality of life for patients with chronic conditions.Read more -
Medical devices-FDA Medical Devices: Definition and Classifications
The FDA defines a medical device as, “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part of accessory which is:recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, orintended to affect the structure or any function of the body of man or other animals, andwhich does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”To determine whether your product meets the FDA definition of a medical device, you should first identify the intended use (what the medical device does) and indications for use (the condition or disease the medical device will diagnose, cure, mitigate, treat, or prevent) of the device. Then, you can see if your device falls under the FDA’s definition.Read more -
Medical devices-Medicines used in combination with a medical device
Before it can issue a CE certificate, the notified body must seek a scientific opinion from EMA on the suitability of the companion diagnostic to the medicinal product concerned if: the medicinal product falls exclusively within the scope of the centralised procedure for the authorisation of medicines, orthe medicinal product is already authorised through the centralised procedure, ora marketing authorisation application for the medicinal product has been submitted through the centralised procedure.For other substances, the notified body can seek the opinion from a national competent authority or EMA.A guidance document is available on the consultation procedure whereby notified bodies seek a scientific opinion from EMA. This is joined by a question-and-answer (Q&A) document on practical arrangements.This guidance should help notified bodies, device manufacturers and medicinal product applicants understand the procedural aspects of the consultation process.The FAQ document below provides an overview of EMA’s line of thinking on issues related to predictive biomarker-guided medicinal product development and assessment involving companion diagnostic (CDx).Medical devices made of substances that are systemically absorbedSome medical devices are made of substances that are absorbed by the human body to achieve their intended purpose.These devices are normally introduced into the human body via an orifice or applied to the skin.Role of EMARead more -
Medical devices-edical Devices; Laboratory Developed Tests
The Food and Drug Administration (FDA, the Agency, or we) is proposing to amend its regulations to make explicit that in vitro diagnostic products (IVDs) are devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act) including when the manufacturer of the IVD is a laboratory. In conjunction with this amendment, FDA is proposing a policy under which FDA intends to phase out its general enforcement discretion approach for laboratory developed tests (LDTs) so that IVDs manufactured by a laboratory would generally fall under the same enforcement approach as other IVDs. FDA is proposing this phaseout to better protect the public health by helping to assure the safety and effectiveness of LDTs. If finalized, this phaseout may also foster the manufacturing of innovative IVDs for which FDA has determined there is a reasonable assurance of safety and effectiveness.Read more -
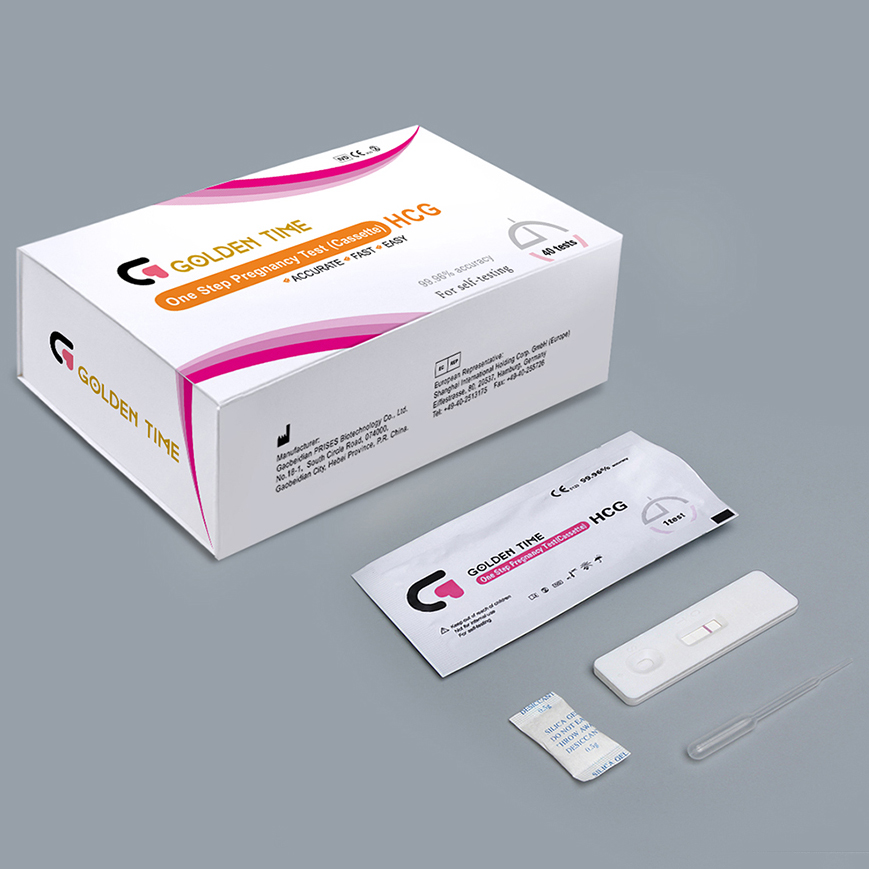
How pregnancy tests work-Pregnancy test
If you think there is a chance you are pregnant, you may wonder when you can take a pregnancy test.You are more likely to get an accurate result if you wait until the day of your missed period or any time after that.There are some very sensitive pregnancy tests that you can do as early as 8 to 10 days after conception (the day you got pregnant).You can take a pregnancy test at home. Your GP or GP practice nurse will also be able to do a pregnancy test for you.Read more -
Pregnancy tests-How does a pregnancy test work?
Home pregnancy tests detect hCG in your urine.There are different types of home pregnancy tests. You can buy these over-the-counter from a pharmacy or supermarket. Some digital kits are more accurate than traditional test kits.There are 2 common methods for home pregnancy tests:holding the end of a chemical dipstick in your urine streamcollecting your urine in a container and then testing itThe dipstick tests for hCG use colours or symbols to show if you are pregnant. For example, they may show a pink or blue line, or a plus sign to show a pregnancy. Digital dipsticks may state the words ‘pregnant’ or ‘not pregnant’ and can be easier to read than symbols.Read more -

How to Take the Most Accurate Pregnancy Test at Home-Pregnancy test
Home pregnancy tests work by detecting a hormone called human chorionic gonadotropin (hCG).1 This is produced by the placenta during pregnancy, and it's found in your urine after a fertilized egg implants in the uterine lining (about six to 12 days after conception). Your body makes increasing amount of hCG as the pregnancy progresses.The presence of hCG leads to a positive pregnancy test result, while lack of hCG causes a negative result. You can also get a false negative by taking the test too soon; this happens if hCG levels didn't rise enough yet to be detected by the test.2What Does a Faint Line on a Pregnancy Test Mean?Read more -

How Early Can You Take a Pregnancy Test?-
For many people, the decision of when to take a pregnancy test can be a major source of anxiety. Sometimes the stress is because you want to be pregnant. Sometimes it’s because you don’t want to be.Regardless of whether you’re hoping for a negative or a positive result, taking an early test may seem like a good way to find out if you’re pregnant right away. Unfortunately, early testing may give you a negative result, even if you are pregnant.It's important to take the test at the right time to maximize your chance of getting an accurate reading. Learn the best time to take a pregnancy test in order to get the most accurate result, as well as the risks associated with testing too early.Read more -
How to take a home pregnancy test-Pregnancy test
All home pregnancy tests measure the amount of a specific hormone known as human chorionic gonadotropin (hCG) in your urine. When the embryo starts to implant in the lining of your uterus, as early as six days after conception, cells that will later develop into the placenta begin to produce hCG.As the placenta grows, the amount of hCG in your body doubles every two days or so during the first few weeks. When hCG levels are high enough, the hormone enters the bloodstream. That's when it starts to show up in the blood and urine. If the home pregnancy test detects enough hCG in your urine, it gives you a positive result.Some tests are more sensitive than others. But in general, it's easier for any home pregnancy test to pick up hCG in the urine when you're 4 weeks pregnant (when there's more hCG) versus 6 days after fertilization (when there's a trace amount of hCG at best).The best way to tell if a test is sensitive enough to pick up the smaller amounts is to read the fine print. Some urine pregnancy tests can detect lower levels of hCG. If you're wanting to test early, before you miss a period, check the package insert to learn more about how much hCG needs to be present in your urine to detect a pregnancy.Read more -

Tests Used to Confirm Pregnancy-pregnancy test kit
Pregnancy testsYou can often tell if you’re pregnant by noticing at least one common symptom. If you’re experiencing any of the symptoms of pregnancy, you should take a home pregnancy test or visit your doctor to confirm the pregnancy.You’ll likely get accurate results from a pregnancy test one day after your first missed period. However, it’s best to wait at least a week after your missed period to be sure that you get the most accurate test results.Home pregnancy testsA home pregnancy test (HPT) can be used on the first day of your missed period. Some very sensitive tests can be used even earlier.These tests work by detecting the hormone human chorionic gonadotropin (hCG) in your urine. This hormone is only found in the body during pregnancy. A chemical in the stick changes color when it comes into contact with this hormone. Waiting times will vary depending on the test, but most take about 10 minutes to deliver an accurate reading.Read more

